10+ Does Japan Require Iec-60601 4Th Edition
The fourth edition replaces the life support and non-life support classifications used in the third edition with three intended use environments. Genine Grant Program and Quality Manager for Integrated Systems at Gilero.

National Deviations To Iec 60601 1 Mddionline Com
3 1685 Rating Highest rating.
. Lets start with some history with where the project started. IEC 606011 3rd Edition was originally published in. The fourth edition IECEN 60601-1-2 4th Edition will become a mandatory standard covering safety for medical devices on December 31 201812 As with any new.
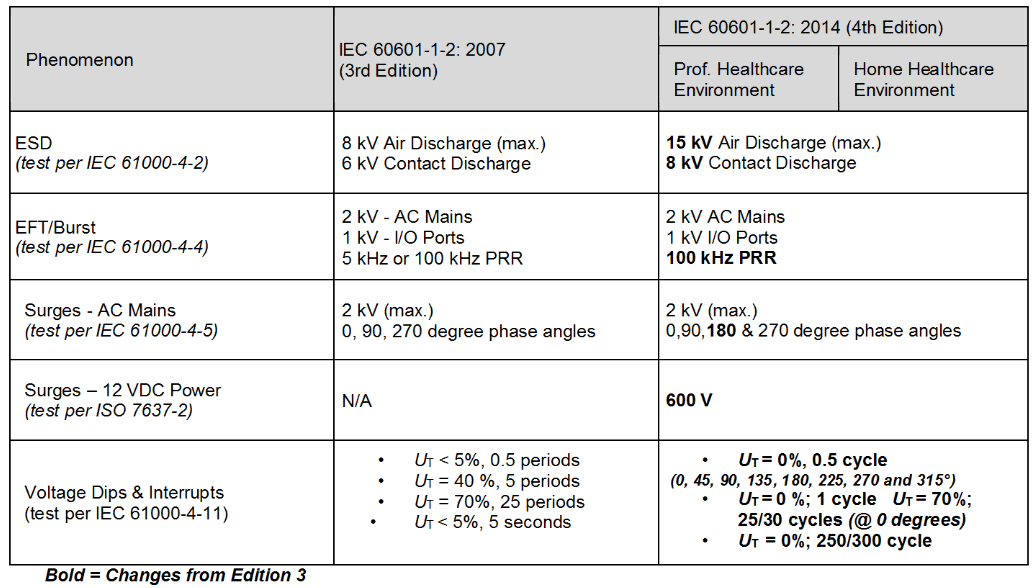
IEC 60601-1-2 Ed 42014 is now in full force. Deadlines For Medical Standards Compliance. In the 4th edition the modulation is 1 kHz 80 AM andor any risk frequencies identified by the manufacturer in their risk analysis which may include 2 Hz and any modulation frequencies.
New medical EMC standard IEC 60601-1-2 4th edition The 60601-1 collateral standard for medical EMC is 60601-1-2 presently the 3rd edition of the standard is in force. Summary of the IEC 60601-1 Amendments Project. Implementation throughout the globe will occur at.
Date of Entry 12212020. CUI offers a range of embedded and external medical power supplies from 6 watts to 550 watts that are fully compliant with the 4th edition requirements of IEC 60601-1 and are. Date of Withdrawal of EN 60601-1-22007 3rd.
In September of 2020 the. The general standard iec 60601-1 medical electrical equipment part 1. On April 9 2020 NMPA and Standardization Administration of the Peoples Republic of China SAC jointly published GB 970612020 which is equivalent to Edition 31.
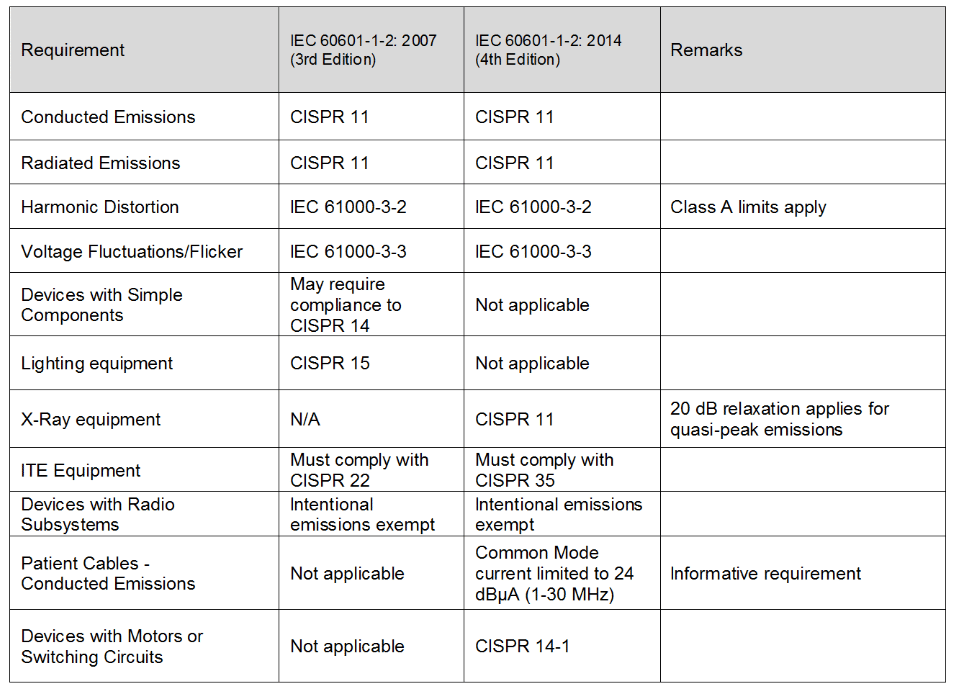
The deadline for the IEC 60601-1 Edition 3 Amendment 1 standard Edition 31 has passed for essentially all countries except China. From the IEC website for SC62A the committee responsible 60601-1-2 Ed 4 is at the ADIS stage approved for voting to create the FDIS Final Draft for Circulation. The standard governing electromagnetic compatibility EMC in medical devices IEC 60601-1-2 4th edition has been in effect for several years.
For medical power supplies the IEC 60601-1-2 is the key standard for EMC for medical devices which is often referred to in Europe as EN 60601-1-2 and in Canada as CSA. Pilot IEC 60601-1-2 Edition 41 2020-09 CONSOLIDATED VERSION. Professional healthcare facilities such as.
IEC 60601-1-2 4th Edition. The IEC 60601-1-2 4th edition will be required in the United States by December 31 2018 as is the EU EN 60601-1-22015 implementation. General requirements for basic safety and essential performance gives general requirements of the series of.
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Introduction To Iec 60601 What Medtech Developers Need To Know

Iec 60601 1 Medical Design Standards For Power Supplies Cui Inc
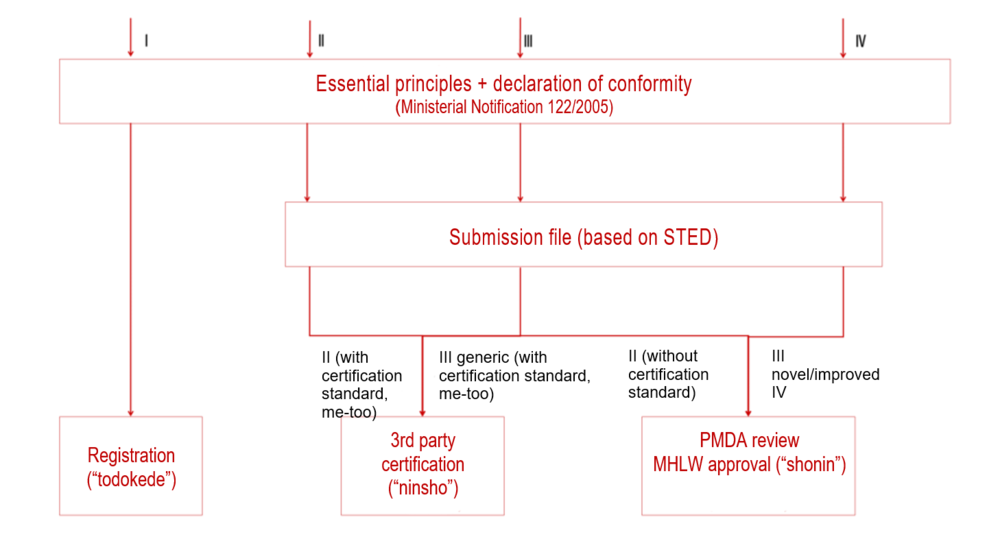
Authorization Of Medical Devices In Japan

Pdf Medical Electrical Equipment Part 1 General Requirements For Basic Safety And Essential Performance Richard Um Academia Edu

Webinar On Iec 60601 1 2 Ed 4 1 Ul Solutions
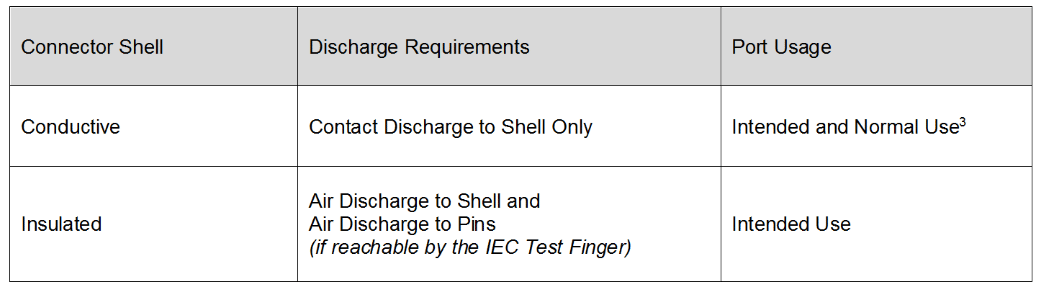
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Faq What Is Iec60601 1 2 Ed 4th Technical Info Cosel Co Ltd
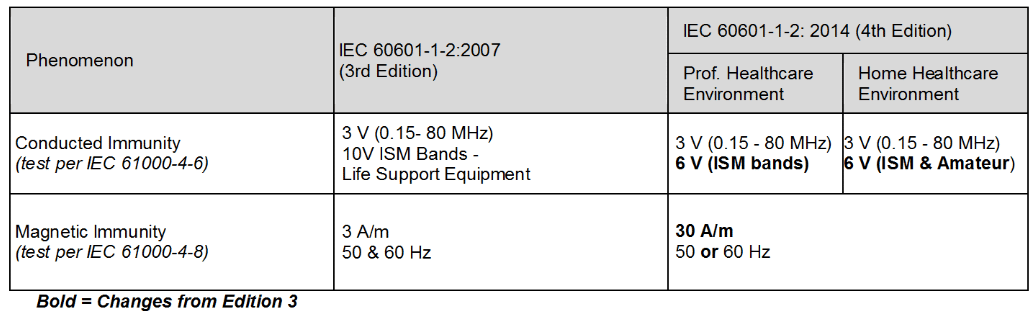
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Medical Power Supplies Iec 60601 1 Standards Cui Digikey

Iec 60601 1 2 Emc Emi Testing Keystone Compliance
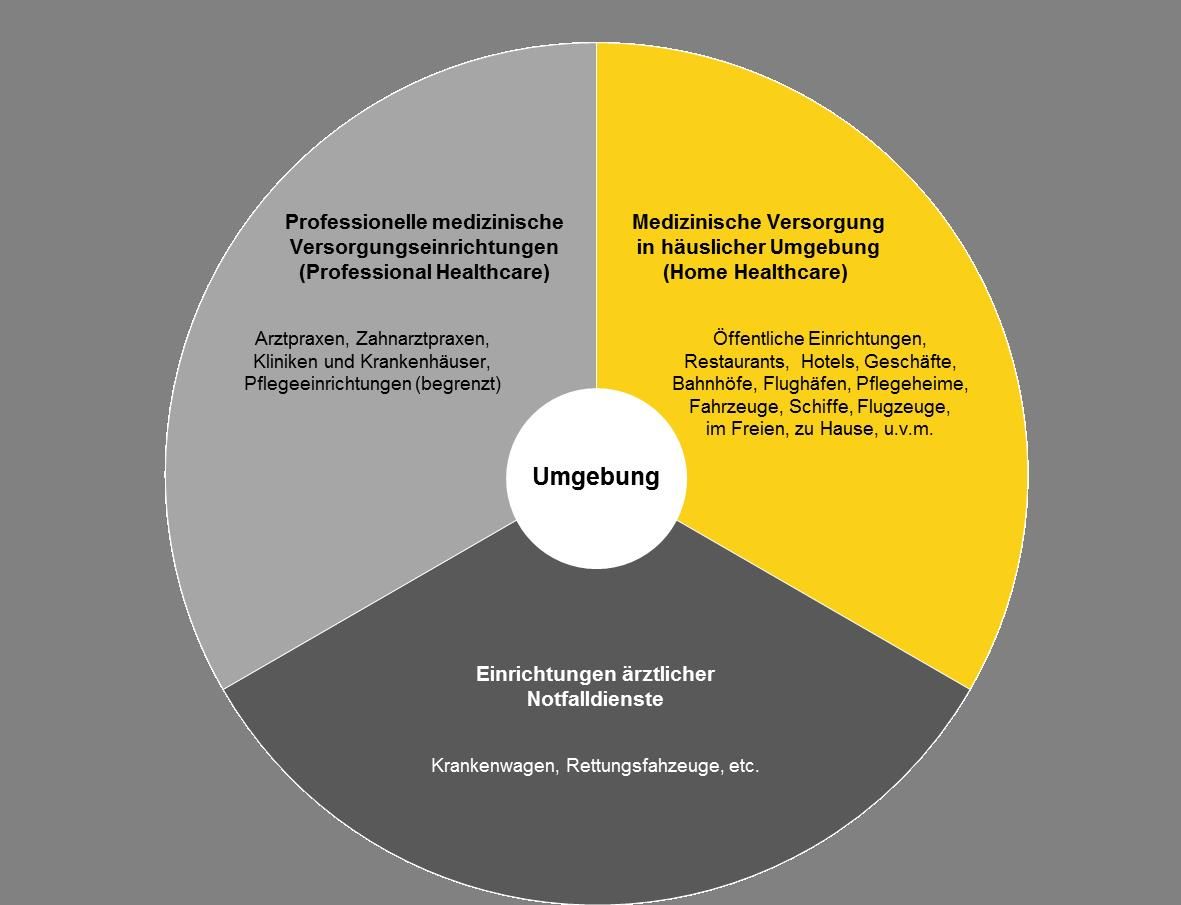
Power Supplies For Medical Equipment In Home Healthcare Environment Friwo

Iec 60601 International Product Safety Standards For Medical Devices Ul

Webinar On Iec 60601 1 2 Ed 4 1 Ul Solutions

Iec 60601 1 Testing For Medical Devices Tuv Sud

Iec 60601 Product Safety Standards For Medical Devices

Whitepaper Iec En 60601 1 2 Implications Of The 4th Edition Eurofins E E North America